The Roivant Sciences strategy of finding shelved drug candidates and castoff research from big pharmaceutical companies has paid off. A compound that Roivant acquired from GlaxoSmithKline now has FDA approval for treating the inflammatory skin condition plaque psoriasis, giving the company its first product that it will commercialize on its own.
The drug, tapinarof, was developed by Roivant subsidiary Dermavant Sciences. That company will market the topical cream under the name “Vtama.” While other topical products are available for treating plaque psoriasis, many of them first reached the market for other indications. Dermavant says its product is the first novel topical drug approved for plaque psoriasis in 25 years. In a prepared statement, Mark Lebwohl of the Icahn School of Medicine at Mount Sinai and lead author of the Phase 3 research that was published in The New England Journal of Medicine, said the Dermavant drug has the potential to become the preferred topical treatment for plaque psoriasis patients.
“Following 20-plus years of minimal innovation in the topical psoriasis treatment space, I believe the approval of Vtama cream is an important step in establishing a new treatment option for adults with mild, moderate and severe plaque psoriasis,” Lebwohl said.
Psoriasis is a chronic condition in which the body’s immune system attacks its own cells. The results of this attack appear prominently on the skin as red and dry patches. The condition affects an estimated 8 million people in the U.S. and up to 125 million people worldwide, according to Dermavant. Plaque psoriasis is the most common form of psoriasis, leading to darker and thicker raised patches on the skin that may be itchy and painful.
Treatments for plaque psoriasis include drugs that work by tamping down the immune system. Topical steroids are widely prescribed for the condition, but long-term use can lead to thinner skin and these drugs lose their efficacy over time. These drugs can also cause problems if used on sensitive areas, such as the face and genitals. Several biologic drugs have approvals in psoriasis, including products from Amgen, AbbVie, Johnson & Johnson, Eli Lilly, and Sanofi. But these injectable medicines circulate systemically, which means that side effects can happen throughout the body. These blockbuster drugs are also very expensive. For both reasons, physicians typically start patients on topical products first and try biologicals only if the disease does not respond.
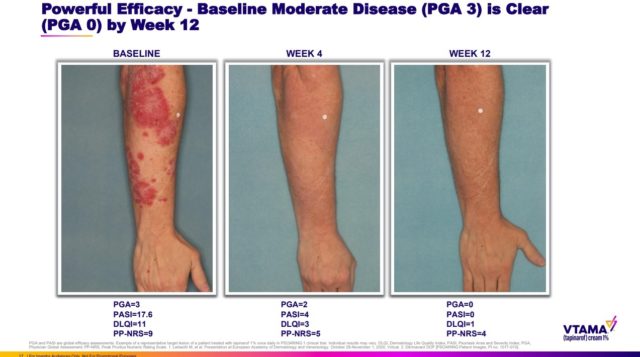
Vtama is a steroid-free cream developed for once-daily application to the skin, including sensitive areas. The drug, a small molecule, is designed to block inflammatory proteins called cytokines. It’s also intended to promote the return of normal skin appearance as well as reduce the oxidative stress that contributes to inflammatory skin conditions. Dermavant said in an investor presentation that its drug could supplant steroids as the standard of care for plaque psoriasis.
The FDA based its approval of the Dermavant drug on the results of two pivotal studies enrolling more than 1,000 total patients. In both studies, the drug met the main goal of showing a statistically significant improvement in skin appearance after 12 weeks of treatment compared against a cream that contained no active ingredient. Adverse events reported in the study were mild to moderate and included inflammation of hair follicles, inflammation in the nose and throat, and contact dermatitis, which is red and itchy skin that appears due to an allergic reaction. Furthermore, the drug has a long-lasting effect once treatment stops with skin clearance continuing for a median of four months.
Dermavant set a $1,325 wholesale price for a tube of Vtama, which is more expensive than some topical plaque psoriasis products currently available, but less than the $1,950 wholesale price of Incyte’s Opzelura, according to the investor presentation. The Incyte product’s approval last September covers atopic dermatitis, a different autoimmune skin condition. Physicians are free to prescribe atopic dermatitis products for treating plaque psoriasis, and they often do. Dermavant said in the presentation that Vtama’s price reflects its differentiated profile and a drug label reflecting the strongest skin clearance effect for a topical plaque psoriasis product.
Though Roivant has diversified its approach to drug development, its initial business model involved scouting for drug assets that were overlooked or stalled at big pharma companies and acquiring their rights. Around each compound, the company sets up subsidiaries around each compound, dubbed “Vants,” each of those units sharing in resources of the parent. Dermavant is one of 14 Vant companies.
In 2018, Dermavant paid GSK £150 million (about $191 million) for global rights to tapinarof, excluding China. With the drug’s U.S. approval, Dermavant is on the hook to pay GSK £100 million (about $133 million). According to the purchase agreement, that payment must happen within 70 days of the approval decision. No further payments, royalties or otherwise, are owed to GSK. However, Dermavant assumed GSK’s obligations to British Columbia-based Welichem Biotech, from which the British pharma giant acquired tapinarof. Under the agreement, Dermavant is responsible for up to 80 million Canadian dollars (about $61 million) in development milestone payments and up to CA$100 million (about $76 million) more tied to commercialization milestones.
Though Vtama marks Roivant’s first drug approval, it is not the first drug from a Vant company to pass regulatory muster. In 2019, Sumitomo Dainippon Pharma of Japan paid $3 billion to acquire five Vants. Those companies have gone on to win FDA approvals in overactive bladder, advanced prostate cancer, uterine fibroid bleeding, and pediatric congenital athymia.
Dermavant has plans for its lead asset that go beyond plaque psoriasis. Last September, the company began a Phase 3 study testing the drug in atopic dermatitis. The program will enroll up to 800 participants, both adults and children with moderate-to-severe disease. Preliminary data from both studies are expected in the first half of next year. Dermavant Chief Medical Officer Philip Brown said that the company is also exploring other potential uses for the drug in dermatology and other immunology indications.
Image from Roivant Sciences investor presentation








