Auris Medical, which racked up a series of clinical trial failures with hearing disorder drugs, is reinventing itself as an RNA technologies company. The company has acquired Trasir Therapeutics, a biotech developing technology that could overcome the challenges of delivering RNA therapies to specific tissues in the body.
The acquisition, a stock deal, closed Tuesday. Samuel Wickline, the founder and majority shareholder of Trasir, has been appointed Auris’s chief scientific officer.
Auris was founded in 2003 and went public in 2014, adopting the fitting stock symbol “EARS.” The then Zug, Switzerland-based company had a pipeline of clinical-stage compounds for hearing disorders such as tinnitus and Meniere’s disease. Auris’s tinnitus drug candidate failed in Phase 3 testing in 2016, then failed again two years later. None of the other Auris programs have led to a successful hearing loss product.
Last fall, Bermuda-based Auris began exploring “strategic alternatives,” corporate lingo for finding a buyer for the company or a merger deal that could take the business in a different direction. In an investor presentation, Auris said Trasir was the most attractive option. The global market for RNA therapies is growing, investors are now familiar with RNA delivery technology, and Trasir’s science brings the opportunity to be disruptive and high growth. For its part, Trasir was looking for a partner to translate its science into new medicines.
Tampa, Florida-based Trasir’s technology aims to overcome the challenges of delivering oliognucleotides, short strands of synthetic DNA or RNA. The engineered viruses that are currently used to deliver genetic medicines aren’t efficient in the way that they deploy their genetic payload into cells. These viruses are also limited in the types of tissues that they can target. The other approaches, lipid nanoparticles and ligand conjugates, preferentially target the liver and may also pose safety problems.
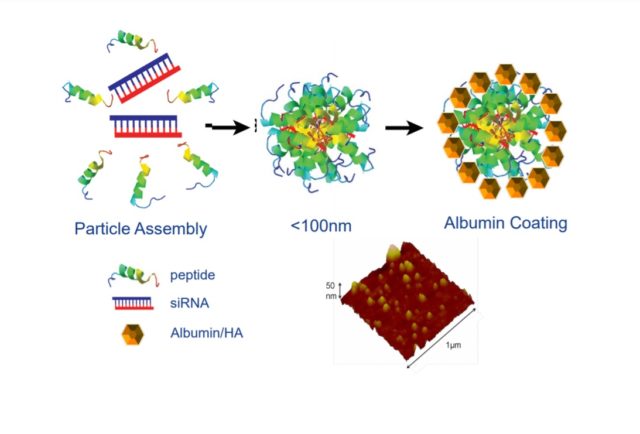
The Trasir technology was licensed from Washington University, where Wickline was researching ways to deliver oligonucleotides safely and effectively to tissues other than the liver. The technology assembles peptides around the RNA, which is in turn encapsulated by an albumin coating, according to the investor presentation. Auris said the technology platform, OligoPhore, can be applied to any type of RNA. This technology enables the systemic delivery of oligonucleotide therapies to target cells other than those in the liver.
In mouse studies testing the delivery of small interfering RNA (siRNA), Auris said that OligoPhore protected the RNA payload from being degraded while it was circulating in the body and also enabled it to be efficiently delivered upon reaching the cellular target. This research also demonstrated that the technology can hit various targets for cancer, rare diseases, and inflammatory disorders.
Trasir’s lead program, AM-401, will initially focus on the delivery of an siRNA therapy, according to the investor presentation. The lead therapeutic indication has not yet been selected, but it will likely be cancer or a rare disease. The next steps include a toxicology study in monkeys. Auris plans to file the regulatory paperwork to begin clinical trials late next year. The company also aims to explore use of the RNA technology for delivery of other types of RNA therapies and gene-editing constructs.
The new RNA focus of Auris will bring a change to the company’s corporate identity. The hearing loss and allergy programs that had comprised Auris will be spun off or sold. Auris is also proposing a name change to Altamira Therapeutics. Auris’s board of directors plans to hold a shareholder meeting in July to discuss the new name. Auris will also adopt the new stock symbol “CYTO,” which the company said is the root word for “cell“ in ancient Greek.
Image from Auris Medical investor presentation